ULO1: Use electrochemical instrumentation effectively and efficiently to make experimental observations.
My practical reports and the voltammograms obtained from the AfterMath Software demonstrate my ability to use the electrochemical instrumentation correctly. My practical reports also demonstrate my ability to explain the observations.
I found that applying the practical knowledge of this unit was one of the more simpler outcomes to achieve. I had difficulty in understanding the concepts until I did the practical. However, once I had looked through the lab manual and applied the knowledge physically, the concepts started to come together for me.
My practical reports 1 and 2 (linked below) demonstrate my ability to use the electrochemical instruments. The potentiostat was the main piece of equipment that centered the practicals. The first time I used the Aftermath software I was slightly overwhelmed. I had no idea what I was doing, why I was doing it, or what information the results were providing me with. Although I knew what colour clips to put on each electrode and the parameters to apply, it was just memory and I did not understand what I was actually doing.
Once I began to write up the practical report I started to understand what I was doing. I understood the working, reference and counter electrodes and their purpose in the cell. I also began to understand the parameters I was applying and how they affected the outcomes.
The second practical was most useful to understand the equipment I was using. The process of polishing the electrode, running the different scan rates, and analysing the voltammograms allowed me to thoroughly understand what I was doing and why. Although the practical was tedious, it was the best way to understand what I was doing. The questions in the discussion section also challenge my knowledge and forced to look deeper into the concepts.
I did not fully understand some of the questions, as seen in my second practical, where some answers were missing, however, I did feel much more comfortable with the concepts.
Construction of a Ag/AgCl Reference Electrode PDF
Electrochemical Reduction of Ferricyanide at a Platinum Electrode PDF
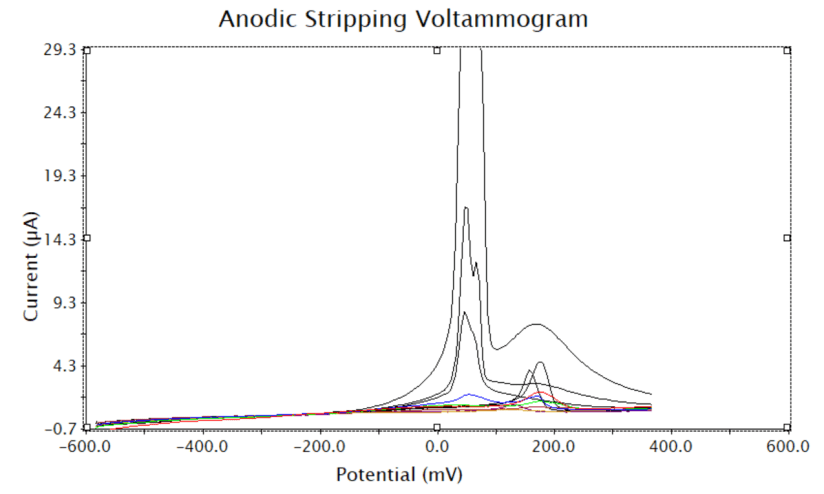
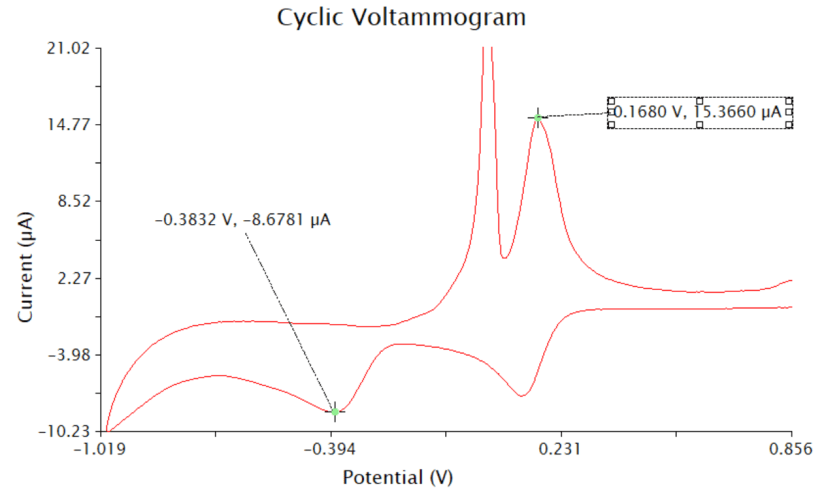
My results from the final practical show that I was able to effectively use the instrumentation. The voltammograms produced were consistent with those produced in other literature, suggesting the instrumentation was used correctly.
All practical reports also show the ability to make observations based on these voltammograms. In practical 3 the Cyclic Voltammetry scan was used to make an observation about what potential to use for the anodic stripping voltammetry. The graph produced two anodic and two cathodic peaks. To determine the peaks that were to obtain the potentials, an understanding of the graphs was required. To interpret the graph an understanding that Cu2+ would be reduced twice needed to be understood, the fact that this would happen at two different potentials also needed to be known.